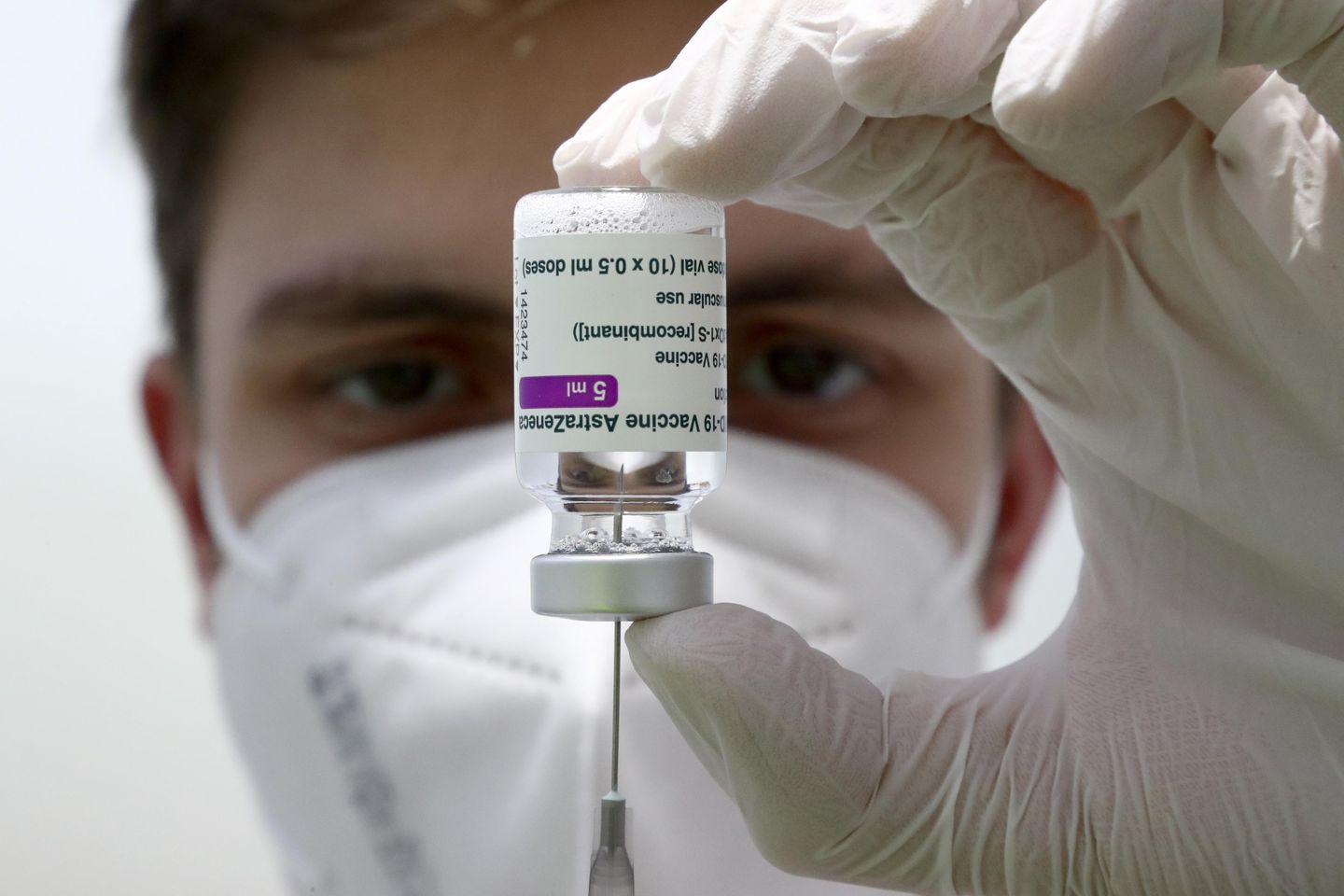
The pharmaceutical company AstraZeneca has announced that it will be withdrawing its COVID-19 vaccine from worldwide circulation and marketing authorizations. This decision comes after concerns about the potential side effects of the vaccine, particularly the rare but serious risk of blood clots. A number of countries had already suspended the use of the AstraZeneca vaccine due to these concerns, and now the company has decided to take further action by completely withdrawing the vaccine from circulation.
The decision to withdraw the AstraZeneca vaccine is a significant development in the global fight against COVID-19. The vaccine had been widely used in many countries as part of their vaccination campaigns, and its withdrawal will likely have an impact on the pace of vaccine distribution and administration. This move by AstraZeneca may also lead to increased scrutiny of other COVID-19 vaccines and their potential side effects, as concerns about safety continue to be a major issue in the vaccination effort.
The decision to withdraw the AstraZeneca vaccine highlights the challenges and complexities of the global vaccination campaign. While vaccines are crucial in controlling the spread of COVID-19 and achieving herd immunity, ensuring their safety and efficacy is essential. The emergence of potential side effects, such as the rare cases of blood clots associated with the AstraZeneca vaccine, has raised questions about the risks and benefits of vaccination. It is important for regulators, health authorities, and pharmaceutical companies to closely monitor vaccine safety and take swift action when concerns arise.
The withdrawal of the AstraZeneca vaccine may also impact the confidence of the public in COVID-19 vaccines in general. The safety and effectiveness of vaccines are key factors in encouraging people to get vaccinated, and any doubts or uncertainties about a particular vaccine can erode trust in the vaccination process. It is crucial for health authorities and experts to communicate openly and transparently about vaccine safety, address concerns promptly, and provide accurate information to the public to maintain confidence in the vaccination effort.
Moving forward, it will be important for countries and health organizations to reassess their vaccination strategies in light of the withdrawal of the AstraZeneca vaccine. This may involve adjusting vaccine distribution plans, prioritizing other vaccines with proven safety records, and addressing any concerns or hesitancy among the public. The global vaccination campaign against COVID-19 remains a critical priority, and ensuring the safety and efficacy of vaccines is essential in overcoming the pandemic. By taking proactive measures and staying vigilant about vaccine safety, countries can continue to make progress in their efforts to control the spread of COVID-19 and protect public health.